Who discovered the electroplating method?
The method known as electroplating is the chemical change of this electrolyte as a result of the electric current given by an external unit that provides direct current support, such as a battery.
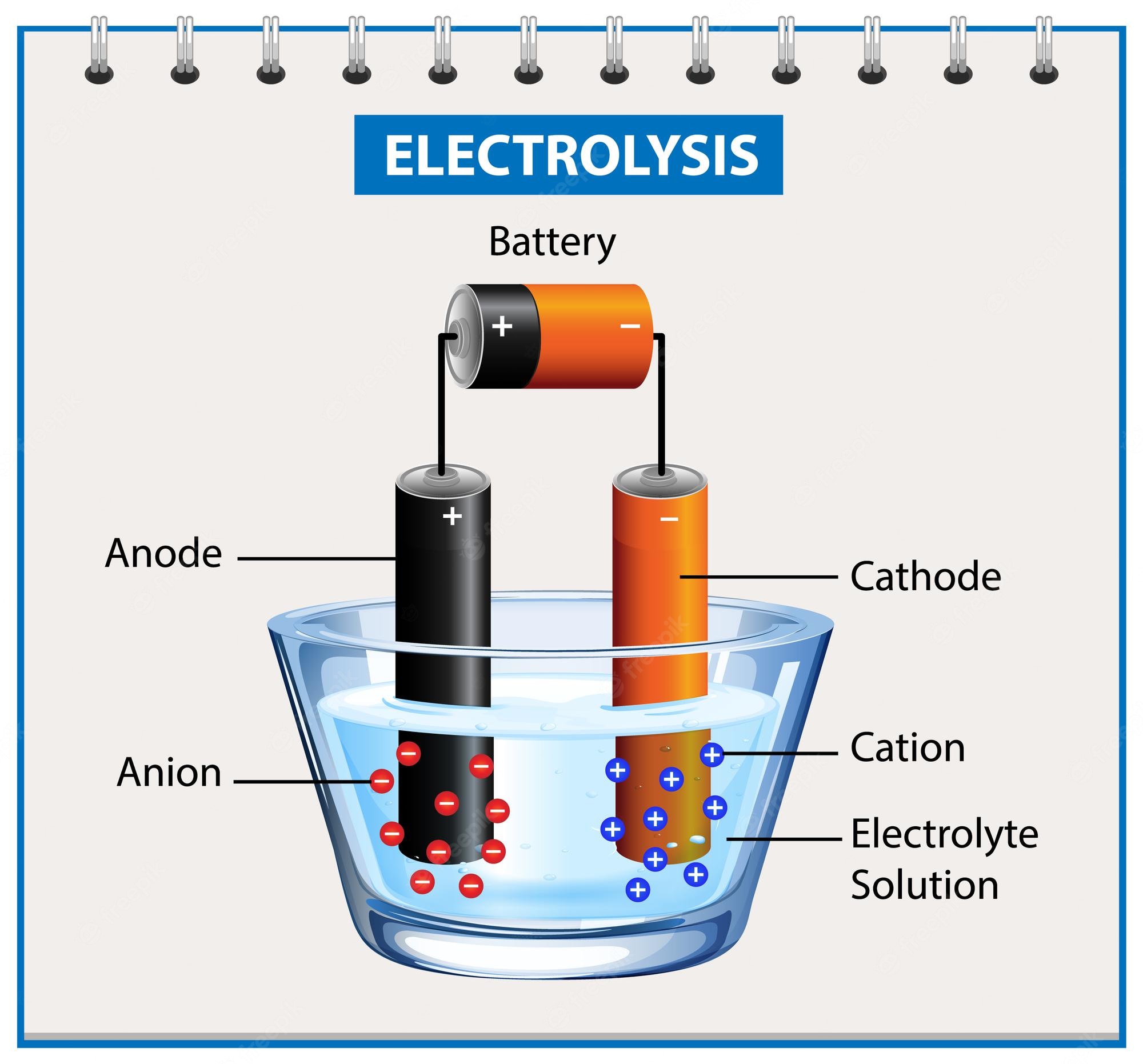
As a result, the metal to be used as the coating material results in the conversion of an electrode called anode to another electrode called cathode through metal ions in the solution. In this way, the target object to which this method is applied is covered with a thin metal layer that forms the anode.
Italian chemist Luigi Brugnatelli (1761 - 1818) invented the electroplating method in 1805. Brugnatelli performed the first electroplating method using Alessandro Volta's battery, previously called the volta battery.
Electrolysis is not a new technology. It was invented in 1800 by William Nicholson and Anthony Carlisle, using voltaic current. Electrolysis was first performed by William Nicholson (1753-1815) and Anthony Carlisle in 1800. Using Alessandro Volta's recently-invented Voltaic pile battery, they immersed two electrodes into water and allowed electricity to flow.
Today, electroplating is used for both decorative and functional purposes, increasing the value and appearance of an object. For example, a piece of jewelry is usually gold plated, and some kitchen utensils, such as cutlery, are plated with cheaper silver. The electroplating technique allows such kitchen tools to be coated with silver. Some coatings, such as zinc and tin, are also used on steel bumpers found in cars. This coating prevents wear of bumpers and some other objects. When car bumpers are first plated with nickel and then chrome, it prevents the abrasions that occur on the bumpers as a result of the change in weather conditions. Hard chrome prevents the wear of moving machine parts as a result of friction.
Electroplating is basically the process of plating a metal onto the other by hydrolysis mostly to prevent corrosion of metal or for decorative purposes. The process uses an electric current to reduce dissolved metal cations to develop a lean coherent metal coating on the electrode.
Electroplating is also used on copper and brass electrical junctions that are plated with silver. Because silver tarnishes much more slowly and is much more conductive than copper and brass, these silver-plated metals become much more efficient electrical junctions. Similarly, the connection parts used in computers are plated with gold or palladium on nickel. In this way, electricity and data transmission between parts becomes more efficient.
